ปฏิกิริยาไอโอดิเนชันของสารอนุพันธ์ 4,6-ไดเมทอกซิอินโดล
Main Article Content
บทคัดย่อ
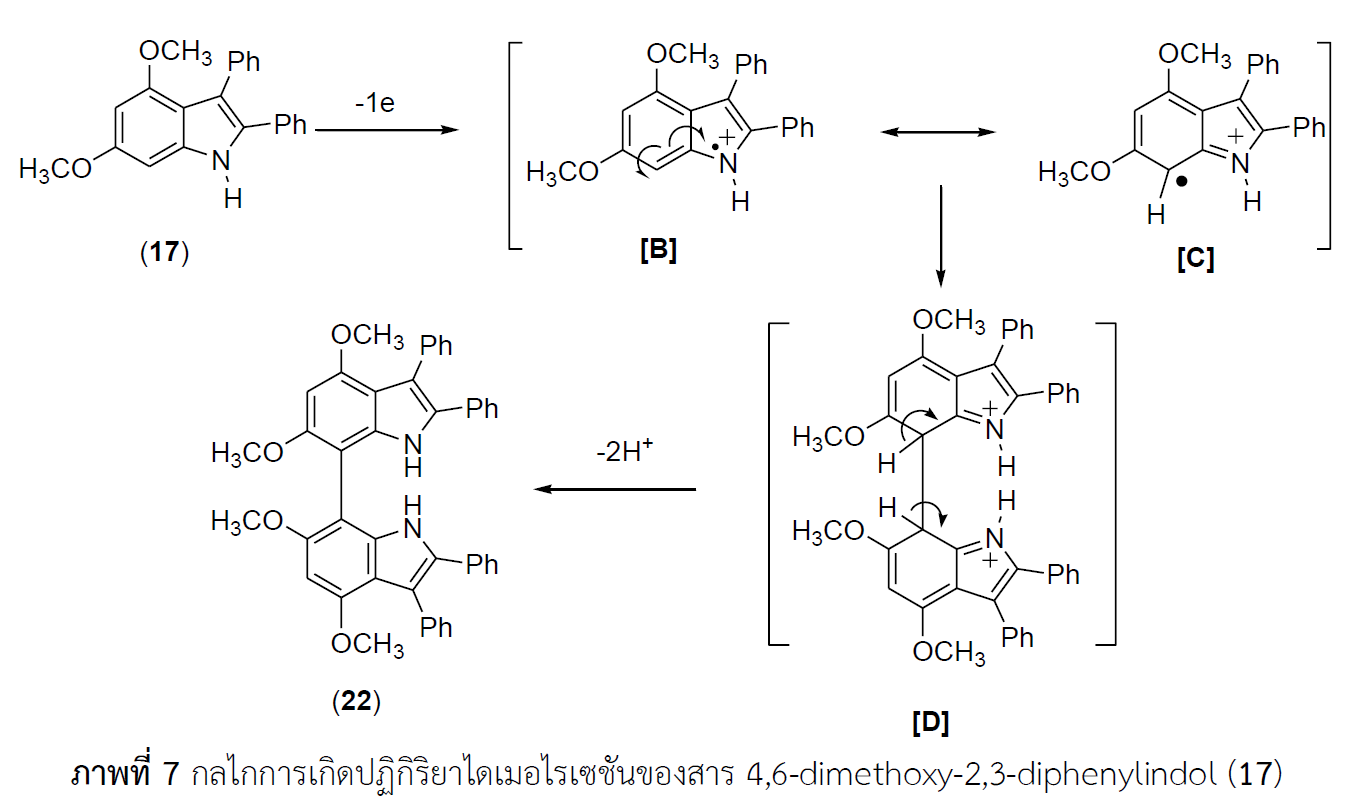
งานวิจัยนี้ได้ศึกษาปฏิกิริยาไอโอดิเนชันของสารอนุพันธ์ 4,6-ไดเมทอกซิอินโดลด้วย เอ็น-ไอโอโดซัคซินาไมด์ในตัวทำละลายไดคลอโรมีเทนที่อุณหภูมิ 0 oC และอุณหภูมิห้อง พบว่าปฏิกิริยาไอโอดิเนชันของสาร 4,6-ไดเมทอกซิ-2,3-ไดฟีนิลอินโดลที่ปราศจากหมู่แทนที่ที่ตำแหน่ง C5 และ C7 และสาร 3-(4-โบรโมฟีนิล)-4,6-ไดเมทอกซิอินโดลที่ปราศจากหมู่แทนที่ที่ตำแหน่ง C2, C5 และ C7 ได้สารอนุพันธ์ 7-ไอโอโดอินโดลซึ่งเป็นสารผลิตภัณฑ์ที่เกิดจากการแทนที่ด้วยอะตอมไอโอดีนที่ตำแหน่ง C7 แต่ในกรณี 7-ฟอร์มิล-4,6-ไดเมทอกซิ-2,3-ไดฟีนิลอินโดลที่ปราศจากหมู่แทนที่ที่ตำแหน่ง C5 ไม่พบผลิตภัณฑ์ 5-ไอโอโดอินโดล นอกจากนี้ปฏิกิริยาไอโอดิเนชันของสาร 4,6-ไดเมทอกซิอินโดลที่ปราศจากหมู่แทนที่ที่ตำแหน่ง C2, C3, C5 และ C7 ก็ไม่พบผลิตภัณฑ์ไอโอโดอินโดลเช่นกัน สุดท้ายเมื่อนำผลจากปฏิกิริยาไอโอดิเนชันไปเปรียบเทียบกับผลของปฏิกิริยาคลอริเนชันและโบรมิเนชัน พบว่าผลการเกิดปฏิกิริยาแบบเลือกสรรในตำแหน่งต่าง ๆ แนวโน้มที่เหมือนกัน แต่ปฏิกิริยาไอโอดิเนชันจะได้ร้อยละของสารผลิตภัณฑ์น้อยกว่าร้อยละผลิตภัณฑ์ของปฏิกิริยาคลอริเนชันและปฏิกิริยาโบรมิเนชัน
Article Details
วารสารวิทยาศาสตร์และวิทยาศาสตร์ศึกษา (JSSE) เป็นผู้ถือลิสิทธิ์บทความทุกบทความที่เผยแพร่ใน JSSE นี้ ทั้งนี้ ผู้เขียนจะต้องส่งแบบโอนลิขสิทธิ์บทความฉบับที่มีรายมือชื่อของผู้เขียนหลักหรือผู้ที่ได้รับมอบอำนาจแทนผู้เขียนทุกนให้กับ JSSE ก่อนที่บทความจะมีการเผยแพร่ผ่านเว็บไซต์ของวารสาร
แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
ทางวารสาร JSSE ได้กำหนดให้มีการกรอกแบบโอนลิขสิทธิ์บทความให้ครบถ้วนและส่งมายังกองบรรณาธิการในข้อมูลเสริม (supplementary data) พร้อมกับนิพนธ์ต้นฉบับ (manuscript) ที่ส่งมาขอรับการตีพิมพ์ ทั้งนี้ ผู้เขียนหลัก (corresponding authors) หรือผู้รับมอบอำนาจ (ในฐานะตัวแทนของผู้เขียนทุกคน) สามารถดำเนินการโอนลิขสิทธิ์บทความแทนผู้เขียนทั้งหมดได้ ซึ่งสามารถอัพโหลดไฟล์บทความต้นฉบับ (Manuscript) และไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนู “Upload Submission” ดังนี้
1. อัพโหลดไฟล์บทความต้นฉบับ (Manuscript) ในเมนูย่อย Article Component > Article Text
2. อัพโหลดไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนูย่อย Article Component > Other
ดาวน์โหลด ไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
เอกสารอ้างอิง
Adams, S.E., Parr, C., Miller D.J., Allemann, R.K. and Hallett, M.B. (2012). Potent inhibition of Ca2+-dependent activation of Calpain-1 by Novel Mercaptoacrylate. Medicinal Chemistry Communications, 3, 566-570.
Black, D.St.C. (1993). New dimensions in indole chemistry: From ligand design to natural product. Synlett, 4, 246-252.
Black, D.St.C., Bowyer, M.C., Kumar, N. and Mitchell, P.S.R. (1993). Calix[3]indoles, new macrocyclic Tris(indolylmethylene) compounds with 2,7-linkages. Journal of Chemical Society, Chemical Communication, 10, 819-821.
Black, D.St.C., Bowyer, M.C., Catalano, M.M., Ivory, A.J., Keller, P.A., Kumar, N. and Nugent, S.J. (1994). Substitution, oxidation and addition reactions at C-7 of activated Indoles. Tetrahedron, 50, 10497-10508.
Black, D.St.C., Kumar, N. and Mitchell, P.S.R. (2002). Synthesis of pyrroloquinolines as indole analogues of flavonols. Journal of Organic Chemistry, 67, 2464-2473.
Hamri, S., Rodriguez, J., Basset, J., Guillaumet, G. and Pujol, M.D. (2012). A convenient iodination of indoles and derivatives. Tetrahedron, 68, 6269-6275.
Hesse, M. (2002). Alkaloids nature’s curse or blessing?. Zürich: Wiley-VCH.
Keawin, T. (2005). Synthesis of endoperoxide of anthracene derivatives and their biological activities and chemistry of 4,6-dimethoxyindole derivatives photooxidation and nitration. Doctoral Dissertation. Bangkok: Mahidol University.
Keawin, T., Kotchapradist, P., Promarak, V., Saengsuwan, S., Sudyoadsuk, T. and Jungsuttiwong, S. (2011). Selective bromination of 4,6-dimethoxyindole derivatives. Journal of Science and Technology, Ubon Ratchathani University, 13, 24-32.
Keawin, T. and Sirithip, K. (2016). Chlorination of 4,6-dimethoxyindole derivatives. Journal of Science and Technology, Ubon Ratchathani University, 18, 39-49.
Marsch, N., Jones, P.G. and Lindel, T. (2015). SmI2-mediated dimerization of indolylbutenones and synthesis of the myxobacterial natural product indiacen B. Beilstein Journal of Organic Chemistry, 11, 1700–1706.
Rajasekharan, S.K., Kim, S., Kim, J.C. and Lee, J. (2020). Nematicidal Activity of 5-iodoindole against root-knot nematodes. Pesticide Biochemistry and Physiology, 163, 76-83.
Tidwell, J.H., Peat, A.J. and Buchwald, S.L. (1994). Synthesis and reactions of 3-(bromomethyl)-1-carbethoxy-4-iodoindole: The preparation of 3,4-differentially substituted indoles. Journal of Organic Chemistry, 59, 7164-7168.
Wu, Y.S., Coumar, M.S., Chang, J.Y., Sun, H.Y., Kuo, F.M., Kuo, C.C., Chen, Y.J., Chang, C.Y., Hsiao, C.L., Liou, J.P., Chen, C.P., Yao, H.T., Chiang, Y.K., Tan, U.K., Chen, C.T., Chu, C.Y., Wu, S.Y., Yeh, T.K., Lin, C.Y. and Hsieh, H.P. (2009). Synthesis and evaluation of 3-aroylindoles as anticancer agents: metabolite approach. Journal of Medicinal Chemistry, 52, 4941-4945.
Zhang, Q., Peng, Y., Wang, X.I., Keenan, S.M., Arora, S. and Welsh, W.J. (2007). Highly potent triazole-based tubulin polymerization inhibitors. Journal of Medicinal Chemistry, 50, 749-754.