การศึกษาสารเชิงซ้อนของโลหะกับกรดลิวอิส Pt(PB) และ Pt(PAl) สำหรับการสลายพันธะของไฮโดรเจนและการเติมไฮโดรเจนแก่เอทิลีนด้วยวิธีทางทฤษฎีฟังก์ชันนัลความหนาแน่น
Main Article Content
บทคัดย่อ
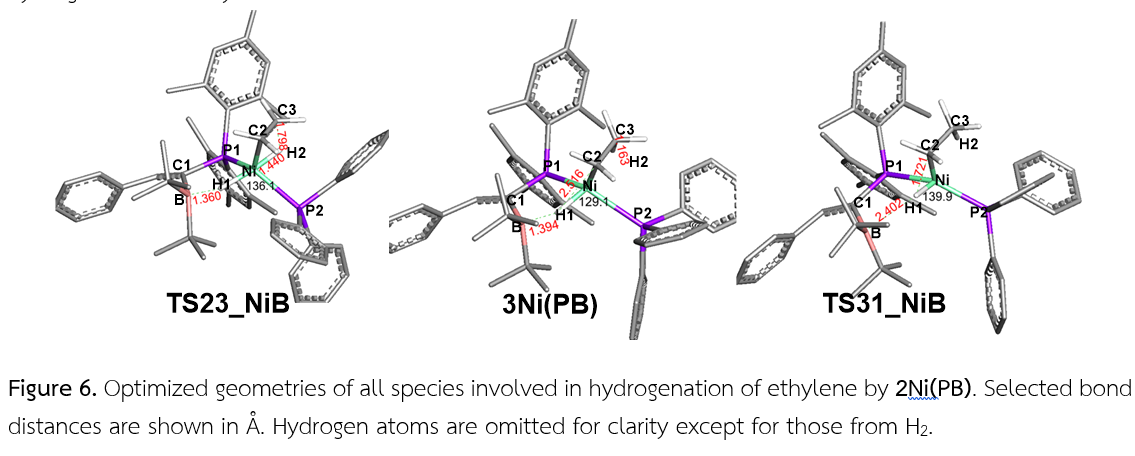
โลหะทรานซิชันหมู่ 10 (M = Pt, Ni) สามารถทำงานร่วมกับกรดลิวอิส (Z = B, Al) เพื่อสลายพันธะของไฮโดรเจนได้ การศึกษาด้วยวิธีทางทฤษฎีฟังก์ชันนัลความหนาแน่นแสดงให้เห็นว่า สารเชิงซ้อน 1Pt(PAl) ซึ่งประกอบด้วยลิแกนด์ Mes2PC(=CHPh)AltBu2 (PAl) เกาะกับโลหะแพลตตินัมสามารถทำงานร่วมกันในการสลายพันธะของไฮโดรเจนผ่านทรานซิชันสเตท TS12_PtAl ซึ่งมีโครงสร้างเป็นแบบตัวที ด้วยพลังงานกระตุ้นอิสระเท่ากับ 31.6 kcal/mol สอดคล้องกับผลการทดลองที่พบว่า สารเชิงซ้อนนี้สามารถสลายพันธะของไฮโดรเจนได้ที่อุณหภูมิ 80 oC จากนั้นเราได้แทนที่อลูมิเนียมซึ่งทำหน้าที่ของกรดลิวอิสด้วยโบรอน โดย 1Pt(PB) ต่างจาก 1Pt(PAl) ตรงที่ไม่พบอันตรกิริยาระหว่าง Pt—B (3.154 Å) 1Pt(PB) จึงมีความไวต่อการสลายพันธะของไฮโดรเจนมากขึ้น ด้วยพลังงานกระตุ้นอิสระลดลงเป็น 21.8 kcal/mol เมื่อมีการใช้โลหะนิกเกิลซึ่งมีความเป็นอิเล็กโตรโพสิทีฟแทนที่แพลตตินัมสำหรับ 1Ni(PB) แทนที่ 1Pt(PB) พบว่า มีความไวต่อปฏิกิริยาการสลายพันธะของไฮโดรเจนมากขึ้นอีก ด้วยพลังงานกระตุ้นอิสระเพียง 9.7 kcal/mol ในขณะที่ทรานซิชัยสเตท TS12_PtB ของสารเชิงซ้อนแพลตตินัมมีโครงสร้างแบบตัวที ทรานซิชันสเตท TS12_NiB ของสารเชิงซ้อนนิกเกิลมีโครงสร้างใกล้กับเตตระฮีดรอน เราได้ทำการวิเคราะห์พลังงานกระตุ้นอิสระด้วยการแยกพิจารณาเป็นส่วนของพลังงานอันตรกิริยาระหว่างสารเชิงซ้อนกับไฮโดรเจน และส่วนของพลังงานที่ใช้การปรับโครงสร้างจากสารเชิงซ้อนในรูปอิสระไปเป็นโครงสร้างสารเชิงซ้อนในรูปที่มีไฮโดรเจนกำลังแตกพันธะที่ทรานซิชันสเตท สารผลิตภัณฑ์ที่ได้จากการสลายพันธะของไฮโดรเจนสามารถเติมไฮโดรเจนแก่เอทิลีนได้ โดยขั้นกำหนดปฏิกิริยา คือ ขั้นตอนที่มีการถ่ายโอนไฮโดรเจนครั้งที่หนึ่งจาก 2M(PZ) ไปยังคาร์บอนของเอทิลีนผ่านทรานซิชันสเตท TS23_MZ ในขั้นการเติมไฮโดรเจนแก่เอทิลีนนี้ 1Ni(PB) อาศัยพลังงานกระตุ้นอิสระเท่ากับ 26.3 kcal/mol ซึ่งเป็นค่าที่ต่ำสุดเทียบกับสารเชิงซ้อนโลหะอื่นในการศึกษานี้ แผนภูมิพลังงานจากการศึกษาด้วยวิธีทางคอมพิวเตอร์ในงานวิจัยนี้ใช้เป็นแนวทางการพัฒนาสารเชิงซ้อนของโลหะกับกรดลิวอิสที่มีความไวต่อปฏิกิริยาได้ต่อไป
Article Details
วารสารวิทยาศาสตร์และวิทยาศาสตร์ศึกษา (JSSE) เป็นผู้ถือลิสิทธิ์บทความทุกบทความที่เผยแพร่ใน JSSE นี้ ทั้งนี้ ผู้เขียนจะต้องส่งแบบโอนลิขสิทธิ์บทความฉบับที่มีรายมือชื่อของผู้เขียนหลักหรือผู้ที่ได้รับมอบอำนาจแทนผู้เขียนทุกนให้กับ JSSE ก่อนที่บทความจะมีการเผยแพร่ผ่านเว็บไซต์ของวารสาร
แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
ทางวารสาร JSSE ได้กำหนดให้มีการกรอกแบบโอนลิขสิทธิ์บทความให้ครบถ้วนและส่งมายังกองบรรณาธิการในข้อมูลเสริม (supplementary data) พร้อมกับนิพนธ์ต้นฉบับ (manuscript) ที่ส่งมาขอรับการตีพิมพ์ ทั้งนี้ ผู้เขียนหลัก (corresponding authors) หรือผู้รับมอบอำนาจ (ในฐานะตัวแทนของผู้เขียนทุกคน) สามารถดำเนินการโอนลิขสิทธิ์บทความแทนผู้เขียนทั้งหมดได้ ซึ่งสามารถอัพโหลดไฟล์บทความต้นฉบับ (Manuscript) และไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนู “Upload Submission” ดังนี้
1. อัพโหลดไฟล์บทความต้นฉบับ (Manuscript) ในเมนูย่อย Article Component > Article Text
2. อัพโหลดไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนูย่อย Article Component > Other
ดาวน์โหลด ไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
เอกสารอ้างอิง
Appelt, C., Westenberg, H., Bertini, F., Ehlers, A. W., Slootweg, J. C., Lammertsma, K., & Uhl, W. (2011). Geminal Phosphorus/Aluminum-Based Frustrated Lewis Pairs: C-H versus C-C Activation and CO2 Fixation. Angewandte Chemie International Edition, 50(17), 3925-3928. doi:10.1002/anie.201006901
Bergner, A., Dolg, M., Küchle, W., Stoll, H., & Preuß, H. (1993). Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Molecular Physics, 80(6), 1431-1441. doi:10.1080/00268979300103121
Chai, J.-D., & Head-Gordon, M. (2008a). Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Physical Chemistry Chemical Physics, 10(44), 6615-6620. doi:10.1039/B810189B
Chai, J.-D., & Head-Gordon, M. (2008b). Systematic optimization of long-range corrected hybrid density functionals. Journal of Chemical Physics, 128(8), 084106. doi:10.1063/1.2834918
Devillard, M., Declercq, R., Nicolas, E., Ehlers, A. W., Backs, J., Saffon-Merceron, N., . . . Bourissou, D. (2016). A Significant but Constrained Geometry Pt→Al Interaction: Fixation of CO2 and CS2, Activation of H2 and PhCONH2. Journal of the American Chemical Society, 138(14), 4917-4926. doi:10.1021/jacs.6b01320
Hariharan, P. C., & Pople, J. A. (1973). The influence of polarization functions on molecular orbital hydrogenation energies. Theoretica chimica acta, 28(3), 213-222. doi:10.1007/bf00533485
Harman, W. H., Lin, T.-P., & Peters, J. C. (2014). A d10 Ni–(H2) Adduct as an Intermediate in H-H Oxidative Addition across a Ni-B Bond. Angewandte Chemie International Edition, 53(4), 1081-1086. doi:10.1002/anie.201308175
Harman, W. H., & Peters, J. C. (2012). Reversible H2 Addition across a Nickel–Borane Unit as a Promising Strategy for Catalysis. Journal of the American Chemical Society, 134(11), 5080-5082. doi:10.1021/ja211419t
Holschumacher, D., Bannenberg, T., Hrib, C. G., Jones, P. G., & Tamm, M. (2008). Heterolytic Dihydrogen Activation by a Frustrated Carbene–Borane Lewis Pair. Angewandte Chemie International Edition, 47(39), 7428-7432. doi:10.1002/anie.200802705
Karunananda, M. K., & Mankad, N. P. (2017). Cooperative Strategies for Catalytic Hydrogenation of Unsaturated Hydrocarbons. ACS Catalysis, 7(9), 6110-6119. doi:10.1021/acscatal.7b02203
Khusnutdinova, J. R., & Milstein, D. (2015). Metal–Ligand Cooperation. Angewandte Chemie International Edition, 54(42), 12236-12273. doi:10.1002/anie.201503873
Frisch, M.J., et al. (2009). Gaussian 09, Revision B.01. Gaussian, Inc., Wallingford.
Marenich, A. V., Cramer, C. J., & Truhlar, D. G. (2009). Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. Journal of Physical Chemistry B, 113(18), 6378-6396. doi:10.1021/jp810292n
Morris, R. H. (2015). Exploiting Metal–Ligand Bifunctional Reactions in the Design of Iron Asymmetric Hydrogenation Catalysts. Accounts of Chemical Research, 48(5), 1494-1502. doi:10.1021/acs.accounts.5b00045
Noyori, R., Yamakawa, M., & Hashiguchi, S. (2001). Metal−Ligand Bifunctional Catalysis: A Nonclassical Mechanism for Asymmetric Hydrogen Transfer between Alcohols and Carbonyl Compounds. The Journal of Organic Chemistry, 66(24), 7931-7944. doi:10.1021/jo010721w
Petersson, G. A., & Al‐Laham, M. A. (1991). A complete basis set model chemistry. II. Open‐shell systems and the total energies of the first‐row atoms. Journal of Chemical Physics, 94(9), 6081-6090. doi:10.1063/1.460447
Petersson, G. A., Bennett, A., Tensfeldt, T. G., Al‐Laham, M. A., Shirley, W. A., & Mantzaris, J. (1988). A complete basis set model chemistry. I. The total energies of closed‐shell atoms and hydrides of the first‐row elements. Journal of Chemical Physics, 89(4), 2193-2218. doi:10.1063/1.455064
Phipps, M. J. S., Fox, T., Tautermann, C. S., & Skylaris, C.-K. (2015). Energy decomposition analysis approaches and their evaluation on prototypical protein–drug interaction patterns. Chemical Society Reviews, 44(10), 3177-3211. doi:10.1039/C4CS00375F
Rokob, T. A., & Pápai, I. (2013). Hydrogen Activation by Frustrated Lewis Pairs: Insights from Computational Studies. In G. Erker & D. W. Stephan (Eds.), Frustrated Lewis Pairs I: Uncovering and Understanding (pp. 157-211). Berlin, Heidelberg: Springer Berlin Heidelberg.
Skara, G., De Vleeschouwer, F., Geerlings, P., De Proft, F., & Pinter, B. (2017). Heterolytic Splitting of Molecular Hydrogen by Frustrated and Classical Lewis Pairs: A Unified Reactivity Concept. Scientific Reports, 7(1), 16024. doi:10.1038/s41598-017-16244-1
Spies, P., Erker, G., Kehr, G., Bergander, K., Fröhlich, R., Grimme, S., & Stephan, D. W. (2007). Rapid intramolecular heterolytic dihydrogen activation by a four-membered heterocyclic phosphane–borane adduct. Chemical Communications (47), 5072-5074. doi:10.1039/B710475H
Tasker, S. Z., Standley, E. A., & Jamison, T. F. (2014). Recent advances in homogeneous nickel catalysis. Nature, 509(7500), 299-309. doi:10.1038/nature13274
Tsoureas, N., Kuo, Y.-Y., Haddow, M. F., & Owen, G. R. (2011). Double addition of H2 to transition metal–borane complexes: a ‘hydride shuttle’ process between boron and transition metal centres. Chemical Communications, 47(1), 484-486. doi:10.1039/C0CC02245D
Weigend, F., & Ahlrichs, R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Physical Chemistry Chemical Physics, 7(18), 3297-3305. doi:10.1039/B508541A
Welch, G. C., Juan, R. R. S., Masuda, J. D., & Stephan, D. W. (2006). Reversible, Metal-Free Hydrogen Activation. Science, 314(5802), 1124-1126. doi:10.1126/science.1134230
Welch, G. C., & Stephan, D. W. (2007). Facile Heterolytic Cleavage of Dihydrogen by Phosphines and Boranes. Journal of the American Chemical Society, 129(7), 1880-1881. doi:10.1021/ja067961j
Xu, X., & Truhlar, D. G. (2011). Accuracy of Effective Core Potentials and Basis Sets for Density Functional Calculations, Including Relativistic Effects, As Illustrated by Calculations on Arsenic Compounds. Journal of Chemical Theory and Computation, 7(9), 2766-2779. doi:10.1021/ct200234r
Yepes, D., Jaque, P., & Fernández, I. (2018). Hydrogenation of Multiple Bonds by Geminal Aminoborane-Based Frustrated Lewis Pairs. Chemistry – A European Journal, 24(35), 8833-8840. doi:10.1002/chem.201800864
Zeng, G., & Sakaki, S. (2013). Unexpected Electronic Process of H2 Activation by a New Nickel Borane Complex: Comparison with the Usual Homolytic and Heterolytic Activations. Inorganic Chemistry, 52(6), 2844-2853. doi:10.1021/ic301733r