การศึกษาพฤติกรรมการกัดกร่อนของโลหะแมกนีเซียม ไทเทเนียม และเหล็กกล้าไร้สนิมโดยเทคนิคเคมีไฟฟ้า
Main Article Content
บทคัดย่อ
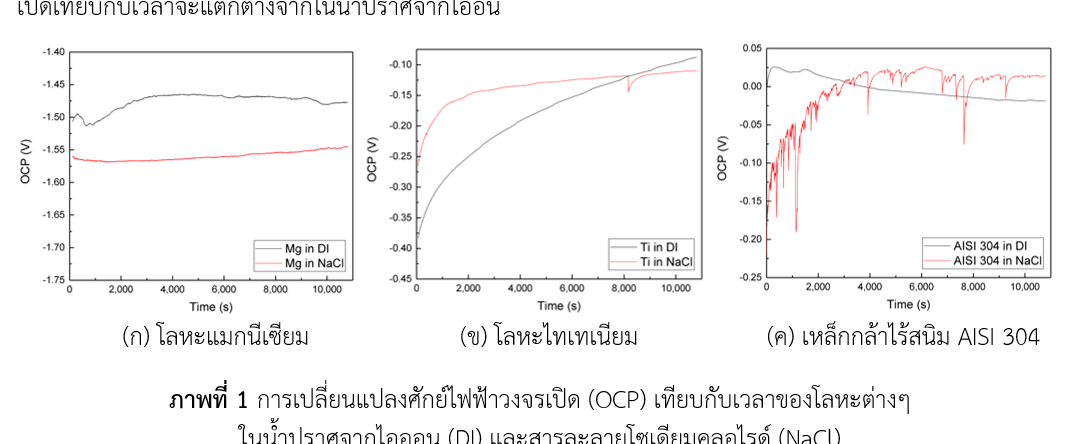
ปัจจุบันแมกนีเซียมอัลลอย ไททาเนียมอัลลอย และเหล็กกล้าไร้สนิมเป็นวัสดุที่ถูกนำมาใช้กันอย่างแพร่หลาย โดยเฉพาะในอุตสาหกรรมยานยนต์และอากาศยาน เนื่องจากมีน้ำหนักเบา และมีความแข็งแรงต่อน้ำหนักสูง ทำให้ประหยัดเชื้อเพลิง ลดปัญหามลพิษจากการปล่อยไอเสียของเครื่องยนต์ แต่ยังสามารถรักษาความปลอดภัยของผู้โดยสารได้ แต่ปัญหาที่สำคัญในการใช้งานวัสดุประเภทโลหะคือการกัดกร่อน ในงานวิจัยนี้จึงศึกษารูปแบบการกัดกร่อนของโลหะแมกนีเซียมบริสุทธิ์ ไททาเนียมบริสุทธิ์ และเหล็กกล้าไร้สนิมเกรด AISI 304 โดยใช้เทคนิคเคมีไฟฟ้า 2 วิธี ได้แก่การวัดการเปลี่ยนแปลงศักย์วงจรเปิดเทียบกับเวลา และการศึกษาความสัมพันธ์ระหว่างกระแสไฟฟ้ากับศักย์ไฟฟ้า โดยจะทำการศึกษาการกัดกร่อนในน้ำปราศจากไอออน และในสารละลายที่มีคลอไรด์ไอออนผสมอยู่ ผลการทดลองจากทั้ง 2 วิธีให้ผลไปในทางเดียวกันว่าวัสดุทั้ง 3 ชนิดมีพฤติกรรมการกัดกร่อนที่แตกต่างกันดังต่อไปนี้ โดยแมกนีเซียมจะพบการกัดกร่อนแบบสม่ำเสมอ และการกัดกร่อนแบบสม่ำเสมอนี้จะรุนแรงขึ้นเมื่อในสารละลายมีคลอไรด์ไอออนผสมอยู่ สำหรับไททาเนียมพบว่าในน้ำปราศจากไอออนมีการสร้างฟิล์มเฉื่อยเพื่อป้องกันผิวจากการกัดกร่อน และในสารละลายที่มีคลอไรด์ไอออนผสมอยู่พบการกัดกร่อนแบบ metastable pitting ที่ไม่รุนแรง ส่วนเหล็กกล้าไร้สนิมพบการกัดกร่อนแบบ metastable pitting อย่างรุนแรงเมื่ออยู่ในสารละลายที่มีคลอไรด์ไออนผสมอยู่ นอกจากนี้เมื่อป้อนศักย์ไฟฟ้าเพียงเล็กน้อยให้กับเหล็กกล้าไร้สนิมการกัดกร่อนสามารถพัฒนาไปเป็นการกัดกร่อนแบบ stable pitting corrosion ได้ ซึ่งเทคนิคไฟฟ้าเคมีทั้ง 2 วิธีให้ผลไปในทางเดียวกัน แต่การวัดการเปลี่ยนแปลงศักย์วงจรเปิดเทียบกับเวลาเป็นเทคนิคที่ไม่ต้องให้ศักย์ไฟฟ้าหรือกระแสไฟฟ้าภายนอกเพื่อรบกวนระบบ เทคนิคนี้จึงจัดเป็นการทดสอบโดยไม่ทำลายที่น่าจะสามารถพัฒนาไปใช้ในการตรวจสอบสถานะการกัดกร่อนของโลหะแบบทันที
Article Details
วารสารวิทยาศาสตร์และวิทยาศาสตร์ศึกษา (JSSE) เป็นผู้ถือลิสิทธิ์บทความทุกบทความที่เผยแพร่ใน JSSE นี้ ทั้งนี้ ผู้เขียนจะต้องส่งแบบโอนลิขสิทธิ์บทความฉบับที่มีรายมือชื่อของผู้เขียนหลักหรือผู้ที่ได้รับมอบอำนาจแทนผู้เขียนทุกนให้กับ JSSE ก่อนที่บทความจะมีการเผยแพร่ผ่านเว็บไซต์ของวารสาร
แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
ทางวารสาร JSSE ได้กำหนดให้มีการกรอกแบบโอนลิขสิทธิ์บทความให้ครบถ้วนและส่งมายังกองบรรณาธิการในข้อมูลเสริม (supplementary data) พร้อมกับนิพนธ์ต้นฉบับ (manuscript) ที่ส่งมาขอรับการตีพิมพ์ ทั้งนี้ ผู้เขียนหลัก (corresponding authors) หรือผู้รับมอบอำนาจ (ในฐานะตัวแทนของผู้เขียนทุกคน) สามารถดำเนินการโอนลิขสิทธิ์บทความแทนผู้เขียนทั้งหมดได้ ซึ่งสามารถอัพโหลดไฟล์บทความต้นฉบับ (Manuscript) และไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนู “Upload Submission” ดังนี้
1. อัพโหลดไฟล์บทความต้นฉบับ (Manuscript) ในเมนูย่อย Article Component > Article Text
2. อัพโหลดไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form) ในเมนูย่อย Article Component > Other
ดาวน์โหลด ไฟล์แบบโอนลิขสิทธิ์บทความ (Copyright Transfer Form)
เอกสารอ้างอิง
Bard, A.J., Stratmann, M. and Frankel, G.S. (2003). Encyclopedia of Electrochemistry. Michigan: Weiley.
Caines S., l Khan, F., Zhang, Y. and Shirokoff, J. (2017). Simplified electrochemical potential noise method to predict corrosion and corrosion rate. Journal of Loss Prevention in the Process Industries, 47, 72-84.
Cottis, R.A. (2006). Sources of Electrochemical noise in corroding systems. Russian Journal of Electrochemistry, 42(5), 497-505.
Enos, D.G. and Scribner, L. (1997). The potentiodynamic polarization scan: Technical report 33. Hampshire: Solartron.
Fekry, A.M. (2009). The influence of chloride and sulphate ions on the corrosion behavior of Ti and Ti-6Al-4V alloy in oxalic acid. Electrochimica Acta, 54, 3480-3489.
Feng, X., Lu, X., Zuo, Yu., Zhuang, N. and Chen, D. (2016). The effect of deformation on metastable pitting of 304 stainless steel in chloride contaminated concrete pore solution. Corrosion Science, 103, 223-229.
Inoue, H., Sugahaea, K., Yamamoto, A. and Tsubakino, H. (2001). Corrosion rate of magnesium and its alloys in buffered chloride solutions. Corrosion Science, 44, 603-610.
Mallick, P.A. (2010). Material, Design and Manufacturing for lightweight Vehicles. Cambridge: Woodhead Publishing.
Palit, G.C. and Elayaperumal, K. (1978). Passivity and pitting of corrosion resistant pure metal Ta, Nb, Ti, Zr Cr and Al in Chloride solution. Corrosion Science, 18, 169-179.
Sasaki, K. and Isaacs, H.S. (2004). Origins of Electrochemical noise during pitting corrosion of aluminium. Journal of Electrochemical society, 151, 124-133.
Vargel, C. (2004). Corrosion of aluminium. Boston: Elsevier, 2004.
Xia, D.-H., Ma, C., Song, S., Ma, L., Wang, J., Gao, Z., and Hu, W. (2017). Assessing atmospheric corrosion of metals by a novel electrochemical sensor combining with a thin insulating net using electrochemical noise technique. Sensors and Actuators B: Chemical, 252, 353–358.