Study of Corrosion Behavior of Magnesium, Titanium and Stainless steel by Electrochemical techniques
Main Article Content
Abstract
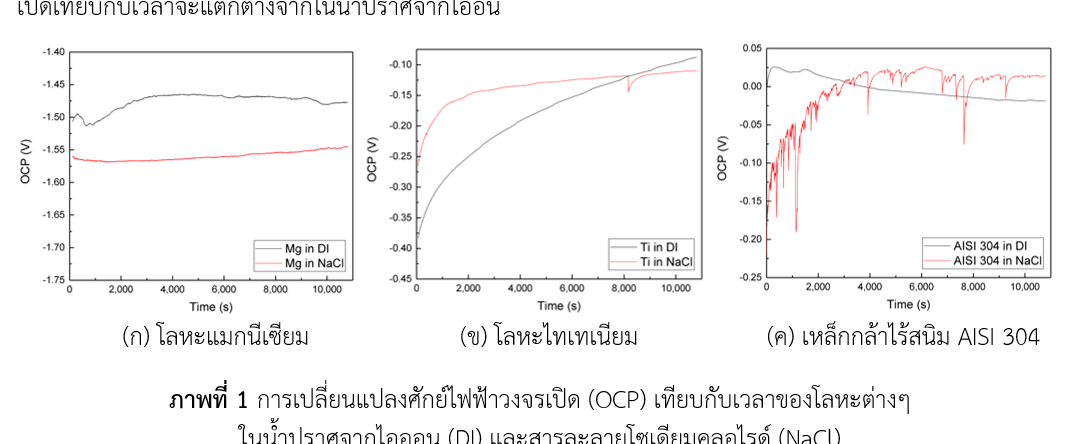
Nowadays, magnesium alloys, titanium alloys and stainless steel are extensively used in automotive and aerospace industries because they are light in weight and exhibit high strength-to-weight ratio. Due to their excellent properties, fuel consumption and exhaust emission of vehicle can be reduced while its safety capability can conserved. However, a major problem in the use of metals in many industries is corrosion. Therefore, this research is aimed at investigating corrosion behavior of pure magnesium, pure titanium and stainless steel AISI 304 in deionized water and solution containing aggressive Cl- ions by using 2 electrochemical techniques: measurement of open circuit potential with time and polarization measurement. It was found that both techniques provided the same results that all 3 materials exhibit different corrosion behavior. Magnesium underwent uniform corrosion in deionized water and this uniform corrosion was escalated into more serious in solution containing chloride ions. For titanium, passive oxide film was formed on its surface when immersion in deionized water but in solution contain chloride ions, its passive surface was attacked by few metastable pitting corrosions. For Stainless steel AISI 304, serious metastable pitting corrosion was observed when it was immersed in solution containing chloride ions and the stable pitting corrosion of stainless steel as well can be easily activated by applying low voltage. Both electrochemical techniques lead to the same results, but measurement of open circuit potential with time requires no external perturbation so it is considered as a nondestructive technique and can be applied to a real time corrosion monitoring system.
Article Details
The Journal of Science and Science Education (JSSE) retain the right of all articles published in JSSE. The coresponding author or the authorized person on behalf of the authors must send the complete Copyright Transfer Form to JSSE before any article get published in JSSE.
Copyright Transfer Form
The JSSE request the coresponding author or the authorized person on behalf of the authors upload the manuscript under the together with the Copyright Transfer Form under the supplementary data. The guidline for uploading both manuscript and Copyright Transfer Form is shown below:
1. Upload the manuscript in the sub-menu, Article Component > Article Text.
2. Upload the the Copyright Transfer Form in the sub-menu, Article Component > Other.
Download Copyright Transfer Form
References
Bard, A.J., Stratmann, M. and Frankel, G.S. (2003). Encyclopedia of Electrochemistry. Michigan: Weiley.
Caines S., l Khan, F., Zhang, Y. and Shirokoff, J. (2017). Simplified electrochemical potential noise method to predict corrosion and corrosion rate. Journal of Loss Prevention in the Process Industries, 47, 72-84.
Cottis, R.A. (2006). Sources of Electrochemical noise in corroding systems. Russian Journal of Electrochemistry, 42(5), 497-505.
Enos, D.G. and Scribner, L. (1997). The potentiodynamic polarization scan: Technical report 33. Hampshire: Solartron.
Fekry, A.M. (2009). The influence of chloride and sulphate ions on the corrosion behavior of Ti and Ti-6Al-4V alloy in oxalic acid. Electrochimica Acta, 54, 3480-3489.
Feng, X., Lu, X., Zuo, Yu., Zhuang, N. and Chen, D. (2016). The effect of deformation on metastable pitting of 304 stainless steel in chloride contaminated concrete pore solution. Corrosion Science, 103, 223-229.
Inoue, H., Sugahaea, K., Yamamoto, A. and Tsubakino, H. (2001). Corrosion rate of magnesium and its alloys in buffered chloride solutions. Corrosion Science, 44, 603-610.
Mallick, P.A. (2010). Material, Design and Manufacturing for lightweight Vehicles. Cambridge: Woodhead Publishing.
Palit, G.C. and Elayaperumal, K. (1978). Passivity and pitting of corrosion resistant pure metal Ta, Nb, Ti, Zr Cr and Al in Chloride solution. Corrosion Science, 18, 169-179.
Sasaki, K. and Isaacs, H.S. (2004). Origins of Electrochemical noise during pitting corrosion of aluminium. Journal of Electrochemical society, 151, 124-133.
Vargel, C. (2004). Corrosion of aluminium. Boston: Elsevier, 2004.
Xia, D.-H., Ma, C., Song, S., Ma, L., Wang, J., Gao, Z., and Hu, W. (2017). Assessing atmospheric corrosion of metals by a novel electrochemical sensor combining with a thin insulating net using electrochemical noise technique. Sensors and Actuators B: Chemical, 252, 353–358.