Hydrogel poly(2-acrylmido-2-methylpropanesulfonic acid-co-acrylic acid) supported copper nanoparticles and their use as catalyst for hydrogen production from hydrolysis of sodium borohydride
Main Article Content
Abstract
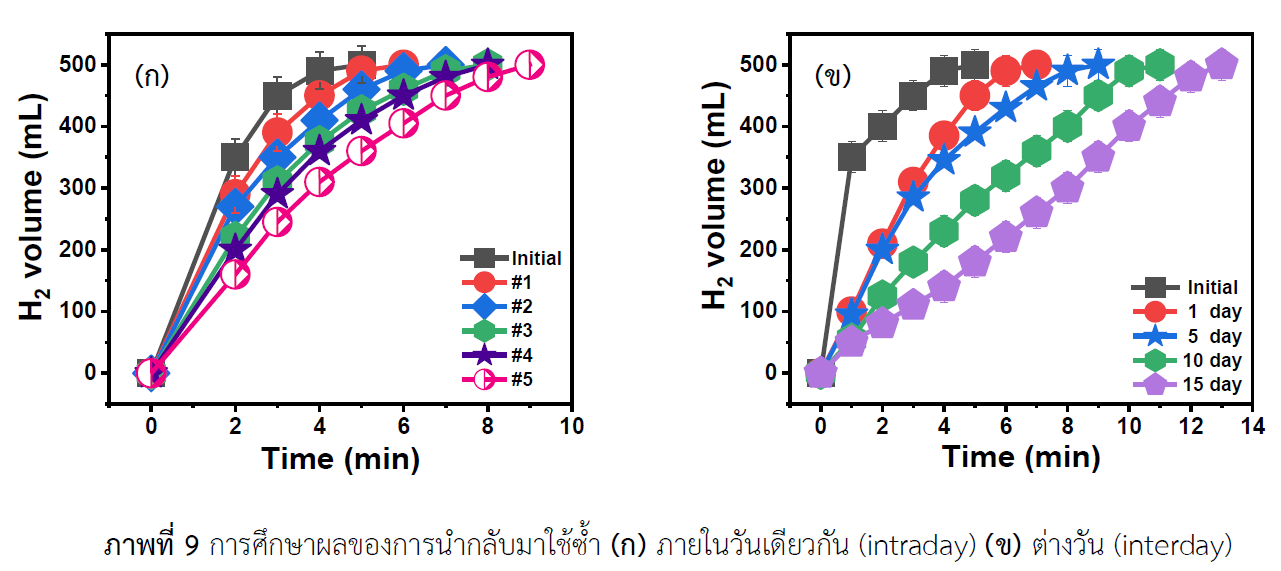
In this work, the hydrogel copolymers from 2-acrylmido-2-methylpropanesulfonic acid (AMPS) and acrylic acid (AA) or Hp(AMPS-co-AA) at different ratios were successfully synthesized by the free radical polymerization for the use in the adsorption of Cu2+ ions through functional groups in the hydrogel network. The bond Cu2+ ions within the hydrogel structure were reduced to the copper nanoparticles (CuNPs) by the reduction reaction using sodium borohydride (NaBH4) as the reducing agent. The swelling of hydrogel was investigated, and Hp(AMPS-co-AA) supported CuNPs were characterized by Fourier transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA). After that, the catalytic activity of Hp(AMPS-co-AA) supported CuNPs was investigated in the hydrolysis of NaBH4 for the hydrogen production. The reaction parameters were calculated by Arrhenius and Eyring equations. It was found that the activation energy (Ea) was 18.20 kJ mol-1; whereas, the activation enthalpy (∆H#) and activation entropy (∆S#) was 20.84 kJ mol-1 and -197.54 kJ mol-1 K-1, respectively. Furthermore, Hp(AMPS-co-AA) supported CuNPs can be reused up to several times without a significant loss of the catalytic activity. This work indicated that Hp(AMPS-co-AA) supported CuNPs were very useful in hydrolysis of NaBH4 for hydrogen production and even in catalysis of other reactions of interest.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The Journal of Science and Science Education (JSSE) retain the right of all articles published in JSSE. The coresponding author or the authorized person on behalf of the authors must send the complete Copyright Transfer Form to JSSE before any article get published in JSSE.
Copyright Transfer Form
The JSSE request the coresponding author or the authorized person on behalf of the authors upload the manuscript under the together with the Copyright Transfer Form under the supplementary data. The guidline for uploading both manuscript and Copyright Transfer Form is shown below:
1. Upload the manuscript in the sub-menu, Article Component > Article Text.
2. Upload the the Copyright Transfer Form in the sub-menu, Article Component > Other.
Download Copyright Transfer Form
References
Ayub, I., Munir, A., Amjad, W., Ghafoor, A. and Nasir, M. S. (2018). Energy- and exergy-based thermal analyses of a solar bakery unit. Journal of Thermal Analysis and Calorimetry, 133, 1001-1013.
Bao, Y., Ma, J. and Li, N. (2011). Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydrate Polymers. 84(1),76-82.
Cao, H., Duan, L., Zhang, Y., Cao, J. and Zhang, K. (2021). Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal transduction and targeted therapy. 6(1), 426.
Ding, J., Li, Q., Su, Y., Yue, Q., Gao, B. and Zhou, W. (2018). Preparation and catalytic activity of wheat straw cellulose based hydrogel-nanometal composites for hydrogen generation from NaBH4 hydrolysis. International Journal of Hydrogen Energy. 43(21), 9978-9987.
Engin, M., and Ozay, O. (2018). The first catalytic hydrolysis of ethylenediamine bisborane with hydrogel-supported metallic nanoparticles. International Journal of Hydrogen Energy. 43(32), 15083-15094.
Güven, O., Demirci, S., Sütekin, S. D., Ari, B. and Sahiner, N. (2022). P(HMA-co-ATU) hydrogel synthesis via gamma radiation and its use for in situ metal nanoparticle preparation and as catalyst in 4-nitrophenol reduction. Radiation Physics and Chemistry, 198, 110217.
Khan, S. B., Ali, F. and Asiri, A. M. (2020). Metal nanoparticles supported on polyacrylamide water beads as catalyst for efficient generation of H2 from NaBH4 methanolysis. International Journal of Hydrogen Energy, 45(3), 1532-1540.
Kojima, Y. (2019). Hydrogen storage materials for hydrogen and energy carriers. International Journal of Hydrogen Energy, 44(33), 18179-18192.
Le, T. T., Pistidda, C., Nguyen, V. H., Singh, P., Raizada, P., Klassen, T. and Dornheim, M. (2021). Nanoconfinement effects on hydrogen storage properties of MgH2 and LiBH4. International Journal of Hydrogen Energy, 46(46), 23723-23736.
Liao, J. and Huang, H. (2020). Magnetic sensitive Hericium erinaceus residue chitin/Cu hydrogel nanocomposites for H2 generation by catalyzing NaBH4 hydrolysis. Carbohydrate polymers. 229, 115426.
Liu, K., Qin, R. and Zheng, N. (2021). Insights into the interfacial effects in heterogeneous metal nanocatalysts toward selective hydrogenation. Journal of the American Chemical Society, 143(12), 4483-4499.
Mahmoud, G. A. (2014). Radiation synthesis of hydrogels as carriers for catalytic nanoparticles and their use in hydrogen production from sodium borohydride. Monatshefte für Chemie-Chemical Monthly, 145, 711-720.
Saeed, A. M. (2013). Temperature effect on swelling properties of commercial polyacrylic acid hydrogel beads. International Journal of Advanced Biological and Biomedical Research, 1(12), 1614-1627.
Sahiner, N. and Seven, F. (2015). The use of superporous p(AAc (acrylic acid)) cryogels as support for Co and Ni nanoparticle preparation and as reactor in H2 production from sodium borohydride hydrolysis. Energy, 71(15), 2014, 170-179.
Sun, Y., Tian, P., Ding, D., Yang, Z., Wang, W., Xin, H., Xu, J. and Han, Y-F. (2019). Revealing the active species of Cu-based catalysts for heterogeneous Fenton reaction. Applied Catalysis B: Environmental. 258, 117985
Ullah, N., Ullah, A and Rasheed, S. (2020). Green synthesis of copper nanoparticles using extract of Dicliptera Roxburghiana, their characterization and photocatalytic activity against methylene blue degradation. Letters in Applied NanoBioScience, 9, 897-901.
Wan, J., Chen, L., Li, Q., Ye, Y., Feng, X., Zhou, A., Long, X., Xia, D. and Zhang, T. C. (2020). A novel hydrogel for highly efficient adsorption of Cu(II): synthesis, characterization, and mechanisms. Environmental Science and Pollution Research, 27, 26621-26630.
Yildiz, S., Nahit A. and Sahiner, N. (2014). Metal nanoparticle-embedded super porous poly (3-sulfopropyl methacrylate) cryogel for H2 production from chemical hydride hydrolysis. International journal of hydrogen energy, 39(27), 14690-14700.
Zhao, L., Li, Q., Su, Y., Yue, Q. and Gua, B. (2017). A novel Enteromorpha based hydrogel for copper and nickel nanoparticle preparation and their use in hydrogen production as catalysts. International Journal of Hydrogen Energy, 42(10), 6746-6756.